Hiperbaria tlenowa wpływa na hipoperfuzje mózgową, zmniejsza czynnik zapalny, stres oksydacyjny oraz dysfunkcję mitochondrialną. Badania oceniające wpływ HBOT wykazały pozytywne zmiany w zachowaniu dzieci po przebytym leczeniu. Hipoperfuzja mózgowa to inaczej zmniejszony przepływ krwi przez mózg, może prowadzić do upośledzenia wymiany substancji pomiędzy krwią a komórkami, w tym glukozy oraz tlenu. Konsekwencją niedoboru tlenu może być martwica oraz niewydolność danego narządu. Hipoperfujza mózgowa powiązana jest z występowaniem pewnych zaburzeń w zachowaniu dzieci z autyzmem, takich jak : powtarzające się zachowania, pragnienie identyczności, zaburzenia wyrażania emocji, zaburzenia mimiczne oraz mowy. Wraz z wiekiem zjawisko to nasila się, dochodzi do zmniejszenia przepływu krwi w mózgu.
Wpływ hiperbarii tlenowej na zaburzenia u dzieci z autyzmem opisał w swoich badaniach Dr. Daniel A Rossignol z Rossignol Medical Center, w Melbourne, USA. Dr. Rossignol od lat zajmuje się tematami związanymi z autyzmem. Od 2006 roku udostępnił wraz z zespołem 55 publikacji dotyczących autyzmu i towarzyszącym chorobie schorzeniom.
Podsumowując, badania naukowe potwierdzają, iż zastosowanie tlenoterapii w autyzmie:
- poprawia u chorych kontakt wzrokowy,
- stymuluje rozwój mowy,
- obniża poziom stresu i poprawia jakość snu,- polepsza umiejętność komunikacji z otoczeniem,
- zmniejsza lękliwość i objawy frustracji,
-poprawia apetyt i trawienie,
- wspomaga walkę z infekcjami bakteryjnymi i pasożytniczymi,
- poprawia dopływ krwi do mózgu,
- redukuje stany zapalne i stres oksydacyjny,
- mobilizuje produkcję komórek macierzystych,
- wpływa pozytywnie na zdolności poznawcze, poprawia pamięć i zdolność koncentracji,
- zwiększa aktywność dziecka i zainteresowanie otoczeniem,
- poprawia umiejętność radzenia sobie z rozwiązywaniem problemów.
外国实际案例
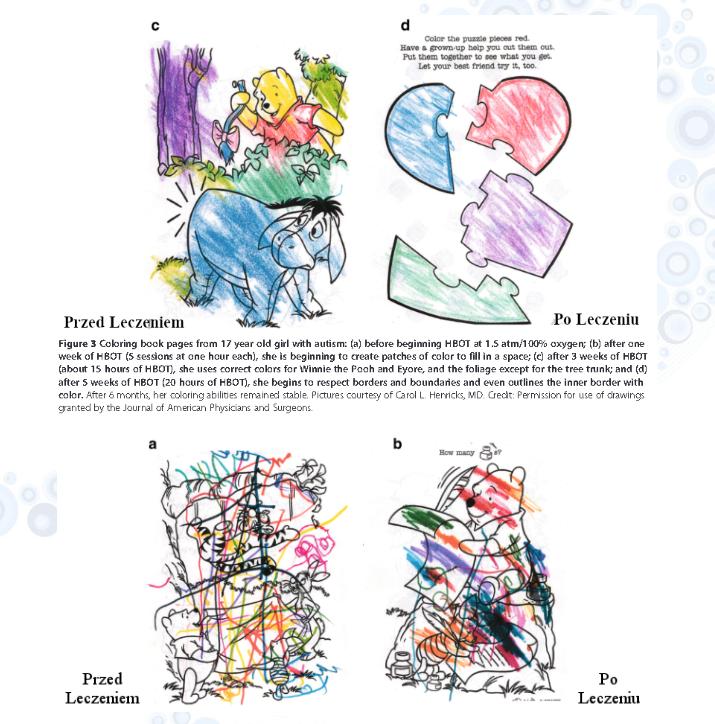
HBOT może wpłynąć pozytywne na rozwój umiejętności manualnych. Dr Carol L. Henricks z Tuscon, Arizona, USA, opisał w 2010 r.
w czasopiśmie „Journal of American Physicians and Surgeons” wpływ terapii HBOT (po serii 20 zabiegów) na umiejętności manualne związane z kolorowaniem u 17-letniej dziewczynki z ASD. Rysunek 3a pokazuje zdolności kolorowania przed przeprowadzoną terapią. Rysunek 3b ukazuje efekty po serii 5 zabiegów 1-godzinnych. Po 15 zabiegach 1-godzinnych zauważono lepszy dobór kolorów oraz większą dokładność w kolorowaniu (rysunek 3c) ,a po 20 zabiegach 1-godzinnych pilnowanie granic przedmiotów (rysunek 3d) [5].
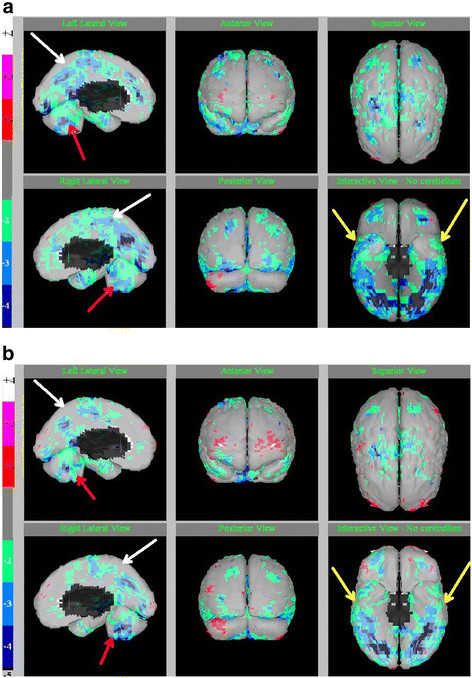
SPECT scan images in a 12 year old boy with autism (a) before and (b) after 80 sessions of HBOT at 1.3 atm. Legend: minus 2 (green) to minus 4 (blue) standard deviations indicate the magnitude of regional hypofunctioning (hypoperfusion). White arrows indicate improvement in deeper cortical hypoperfusion patterns. Red arrows on sagittal slices show the midline cerebellum hypoperfusion and improvements after HBOT. Yellow arrows on the “underside” view show the temporal lobe hypoperfusion with improvements after HBOT. Pictures courtesy of J. Michael Uszler, MD. Credit: Permission for use of figure from Hyperbaric Oxygen for Neurological Disorders granted by Best Publishing Company, Palm Beach Gardens, FL.
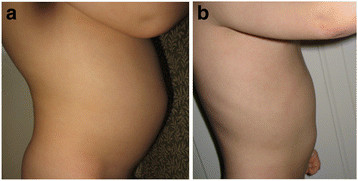
6 year old boy with autism who received HBOT at 1.5 atm. Before HBOT, physical exam reveals distended abdomen (a) with chronic diarrhea. After HBOT, patient has improvements in distended abdomen (b) and bowel movements. Figure use with parental permission. Credit: Permission for use of figure from Hyperbaric Oxygen for Neurological Disorders granted by Best Publishing Company, Palm Beach Gardens, FL.

Handwriting in a 6 year old boy (a) before and (b) after 40 HBOT sessions at 1.3 atm. Pictures courtesy of James Neubrander, MD. Credit: Permission for use of figure from Hyperbaric Oxygen for Neurological Disorders granted by Best Publishing Company, Palm Beach Gardens, FL.
Conclusions
HBOT at the pressures commonly used in children with ASD (up to 1.5 atm/100% oxygen) has been reported to improve cerebral perfusion, decrease markers of inflammation and not worsen oxidative stress markers. Most studies of HBOT in children with ASD reported improvements in several behavioral domains although many of these studies were not controlled. Although the two trials employing a control group reported conflicting results, a recent systematic review noted several important distinctions between these trials. Collectively, the reviewed studies indicate that the use of HBOT in children with ASD is associated with minimal adverse events and is well tolerated. We conclude that HBOT is a safe and potentially effective treatment for children with ASD but that further studies are warranted. Future studies would be wise to use standardized behavioral measurement tools and physiological biomarkers in a controlled study design. Targeting ASD subgroups that possess specific physiological abnormalities with HBOT may be a potentially fruitful method for determining which ASD individuals would benefit from treatment with HBOT.
美国政府官网表明HBOT对自闭症有效果:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3472266/[2]
临床实验1
Abstract
Background
Several uncontrolled studies of hyperbaric treatment in children with autism have reported clinical improvements; however, this treatment has not been evaluated to date with a controlled study. We performed a multicenter, randomized, double-blind, controlled trial to assess the efficacy of hyperbaric treatment in children with autism.
Methods
62 children with autism recruited from 6 centers, ages 2–7 years (mean 4.92 ± 1.21), were randomly assigned to 40 hourly treatments of either hyperbaric treatment at 1.3 atmosphere (atm) and 24% oxygen ("treatment group", n = 33) or slightly pressurized room air at 1.03 atm and 21% oxygen ("control group", n = 29). Outcome measures included Clinical Global Impression (CGI) scale, Aberrant Behavior Checklist (ABC), and Autism Treatment Evaluation Checklist (ATEC).
Results
After 40 sessions, mean physician CGI scores significantly improved in the treatment group compared to controls in overall functioning (p = 0.0008), receptive language (p < 0.0001), social interaction (p = 0.0473), and eye contact (p = 0.0102); 9/30 children (30%) in the treatment group were rated as "very much improved" or "much improved" compared to 2/26 (8%) of controls (p = 0.0471); 24/30 (80%) in the treatment group improved compared to 10/26 (38%) of controls (p = 0.0024). Mean parental CGI scores significantly improved in the treatment group compared to controls in overall functioning (p = 0.0336), receptive language (p = 0.0168), and eye contact (p = 0.0322). On the ABC, significant improvements were observed in the treatment group in total score, irritability, stereotypy, hyperactivity, and speech (p < 0.03 for each), but not in the control group. In the treatment group compared to the control group, mean changes on the ABC total score and subscales were similar except a greater number of children improved in irritability (p = 0.0311). On the ATEC, sensory/cognitive awareness significantly improved (p = 0.0367) in the treatment group compared to the control group. Post-hoc analysis indicated that children over age 5 and children with lower initial autism severity had the most robust improvements. Hyperbaric treatment was safe and well-tolerated.
Conclusion
Children with autism who received hyperbaric treatment at 1.3 atm and 24% oxygen for 40 hourly sessions had significant improvements in overall functioning, receptive language, social interaction, eye contact, and sensory/cognitive awareness compared to children who received slightly pressurized room air.
Trial Registration
clinicaltrials.gov NCT00335790
临床实验2
Abstract
Background
Recently, hyperbaric oxygen therapy (HBOT) has increased in popularity as a treatment for autism. Numerous studies document oxidative stress and inflammation in individuals with autism; both of these conditions have demonstrated improvement with HBOT, along with enhancement of neurological function and cognitive performance. In this study, children with autism were treated with HBOT at atmospheric pressures and oxygen concentrations in current use for this condition. Changes in markers of oxidative stress and inflammation were measured. The children were evaluated to determine clinical effects and safety.
Methods
Eighteen children with autism, ages 3–16 years, underwent 40 hyperbaric sessions of 45 minutes duration each at either 1.5 atmospheres (atm) and 100% oxygen, or at 1.3 atm and 24% oxygen. Measurements of C-reactive protein (CRP) and markers of oxidative stress, including plasma oxidized glutathione (GSSG), were assessed by fasting blood draws collected before and after the 40 treatments. Changes in clinical symptoms, as rated by parents, were also assessed. The children were closely monitored for potential adverse effects.
Results
At the endpoint of 40 hyperbaric sessions, neither group demonstrated statistically significant changes in mean plasma GSSG levels, indicating intracellular oxidative stress appears unaffected by either regimen. A trend towards improvement in mean CRP was present in both groups; the largest improvements were observed in children with initially higher elevations in CRP. When all 18 children were pooled, a significant improvement in CRP was found (p = 0.021). Pre- and post-parental observations indicated statistically significant improvements in both groups, including motivation, speech, and cognitive awareness (p < 0.05). No major adverse events were observed.
Conclusion
In this prospective pilot study of children with autism, HBOT at a maximum pressure of 1.5 atm with up to 100% oxygen was safe and well tolerated. HBOT did not appreciably worsen oxidative stress and significantly decreased inflammation as measured by CRP levels. Parental observations support anecdotal accounts of improvement in several domains of autism. However, since this was an open-label study, definitive statements regarding the efficacy of HBOT for the treatment of individuals with autism must await results from double-blind, controlled trials.
Trial Registration
clinicaltrials.gov NCT00324909